Canola is vulnerable to a number of seed and seedling diseases, including those caused by Rhizoctonia solani, Fusarium species and Pythium species. However, in addition to effective scouting at the right time, there are some preventative and management options to deal with these diseases.
Important tips for best management
- A registered seed treatment with multiple fungicide active ingredients can minimize the threat of a number of seed and seedling diseases that canola is vulnerable to.
- While early seeding is best for yield in general, shallow seeding and good seed to soil contact allows the crop to establish as quickly as possible. Seeds that emerge and grow quickly are less likely to succumb to seedling diseases. By the two- to four-leaf stage, the hypocotyl portion of the root develops a waxy coating called suberin that helps seedlings withstand further infections.
- Start field scouting 10 to 14 days after seeding. Examine non-emerged areas looking for seed as well as dying or dead plants. Typically when plants die prior to emerging the plant remains may be only found for a few days afterward.
Overview
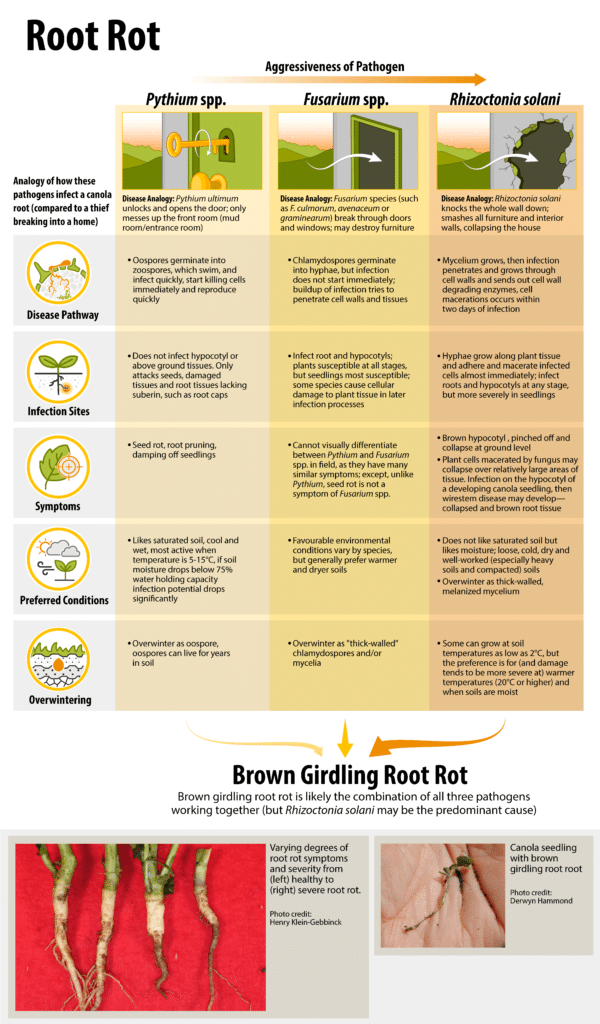
Rhizoctonia solani, Fusarium species and Pythium species are the primary soil-borne pathogens in the seedling disease complex, whose symptoms include seed decay, pre- and post-emergence damping-off (wirestem), seedling blight and seedling root rot. Tighter canola rotations do seem to increase the severity of these diseases, but seed treatments will increase seeding emergence and growth of canola when these pathogens are present 1.
Seed and seedling losses from these diseases tend to be highest under cold wet conditions or when the seedbed is not firmly packed under dry, cool conditions. The disease complex is most often a problem in the northwestern Prairies with prolonged low soil temperatures 2.
More specifically, cold damp soils favor Pythium species. Loose, cold, dry and well-worked soils favor Rhizoctonia solani. Wet, heavy soils favor Fusarium species.
Seed-borne fungi, such as Alternaria species, rarely cause canola emergence problems if seed is properly treated.
If seedling losses are uniform throughout the stand, surviving canola plants can compensate by growing larger without any significant drop in yield. If losses are patchy and large areas die out, then sufficient compensation cannot take place. Reseeding may be the best option for those parts of the field, provided surface moisture and soil temperatures will allow for shallow seeding and rapid emergence of the reseeded crop.
Tighter canola rotations do seem to increase the severity of these diseases, but seed treatments will increase seedling emergence and growth of canola when these pathogens are present 1,3.
Many factors can result in poor emergence that could be mistaken for seedling disease. These include the desiccation of emerging hypocotyls in dry soil, heat damage, wind damage, excessive seed depth, or insect damage from pests like cutworms or wireworms 3.
Learn more about reducing seedling blight to improve stand establishment in hybrid canola and the effect of crop rotation on canola seedling blight and soil pathogen population dynamics.
Disease cycle
All three seedling disease complex pathogens have unique disease cycles, and a description of their general progression is provided below. There is detailed information about each pathogenA disease-causing organism (such as a fungus or bacteria). More in the root rot section.
Dry seeds become vulnerable to attack by these pathogens as soon as they take up water prior to germination. These pathogens grow in the soil when conditions are suitable or are stimulated by secretions from germinating seeds or roots of host plants. These pathogens take advantage of young succulent roots and young stem (hypocotyl) tissue, which are more susceptible to infection. Plants with damaged root systems may stagnate for a while, then grow and mature in the usual way.
Once in the plant, these pathogens multiply causing decay that damages or kills the seedling. They can form microscopic resting bodies within or on the surface of the infected tissue following the death of the cells. The resting or dormant bodies allow the pathogens to survive until another susceptible host becomes available. At the two- to four-leaf stage, the below ground parts of the plants become sufficiently woody to withstand further infections and are able to regenerate more root tissue than they lose.
These pathogens may persist throughout the life of the plant on infected root tissue, reducing the efficiency of the root system in absorbing moisture and nutrients, and reducing tolerance to moisture stress in midsummer heat. This results in a stand with poor vigourSeed properties that determine the potential for rapid uniform emergence and development of normal seedlings under a wide range of field conditions. More and reduced yield.
Influence of environment
Seedling disease complex is dependent on weather conditions and crop rotation. The greatest losses come from seeding early into cold soils and from deep seeding. Soil moisture and temperature influence disease severity. Conditions that favour slow growth and cold damp soils favor Pythium species. Loose, cold, dry and well-worked soils favour Rhizoctonia solani. Wet, heavy soils favour Fusarium species. These generalizations for environmental conditions are not exclusive, and in fact infections do occur for all in a wide range of conditions.
Identification
Symptoms
Symptoms of the seedling disease complex appear as patchy emergence during the four weeks following seeding, or up to the four-leaf stage. Patchy emergence can result from a wide range of factors, including insects, seeding errors, and soil conditions.
When scouting for the cause of poor emergence, check for the following disease symptoms:
- Seeds fail to germinate and become soft and pulpy due to seed decay.
- Seeds germinate but the developing seedlings decay and fail to emerge (due to pre-emergence damping-off).
- Seedlings emerge and appear normal above ground, but either the roots decay with rot moving rapidly up into the hypocotyl, or the young stem, which may be partially or completely girdled with decay and shrivel at any point. In this case, the young above-ground parts of the canola seedling may also exhibit a purple or chlorotic discoloration. When the decay reaches the soil surface, the emerged part of the seedling topples over, wilts and dies. This could be due to seedling blight, wirestem or post-emergence damping-off.
- The hypocotyl appears constricted or shriveled and may be discoloured reddish brown. In moist topsoil, the shriveled stem may persist for a while, but in dry windy conditions, the whole seedling disappears in a few days. Seedlings may also emerge and then stagnate in the two- to four-leaf growth stage even when growing conditions appear favorable. Rootlets appear to be missing. This is seedling root rot.
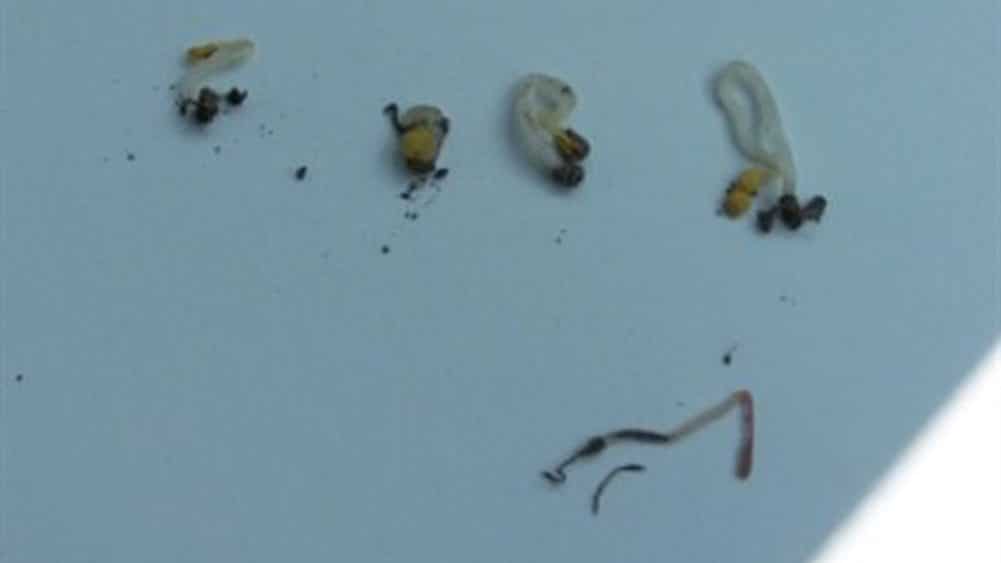
Differentiating between disease and insect damage
Determining if the damage was caused by an insect or disease can be a challenge. Part of a good scouting kit requires a shovel, water bottle to wash soil off roots, and a magnifying glass to check for symptoms. Compare areas where seedlings did not emerge or where plants are tipped over to areas that have strong emergence. Flea beetle and/or cutworm damage may occur together with the disease complex. Look closely at the damage. Flea beetles do not cause the hypocotyl to rot off or constrict at or below the soil line. However, in cool conditions they do eat portions out of the hypocotyl at or above the soil line. Young cutworms chew holes and notches into leaves while older cutworms eat into stems and usually sever them at or just above the soil surface. Wireworms will shred the root, and plants will wither and die. Wireworms are typically found feeding down the seed row on canola and other volunteer cereals. Wirestem symptoms are often the result of deep seeding due to prolonged exposure of the sensitive hypocotyl.
Affected regions
The disease complex is most often a greater problem in the northwestern Prairies with prolonged low soil temperatures, but is not limited to these areas 2.
Seed treatments are, for the most part, very effective against canola seedling diseases caused by Fusarium, Pythium and Rhizoctonia. However, plant pathologists expect that continued frequent use of these seed treatments will select for seedling disease pathogens that can overcome these treatments in the mid to long term 1. Growing canola in a tight rotation may increase the risk of these diseases with more canola volunteers, which are not treated. These volunteer seedlings (which can be numerous in some fields) can act as a host for all seedling diseases. Even though most volunteers in most cases are removed with herbicides, the damage may have already been done when it comes to building up the seedling pathogenA disease-causing organism (such as a fungus or bacteria). More population.
Management
Minimize seedling disease losses
Losses due to seedling diseases can be minimized by using seed treatments, limiting stress on seedlings and taking steps to allow for quick emergence. The seed treatments currently on the market combine up to three fungicide active ingredients, providing protection from a wide range of seed and seedling disease pathogens.
Seeds that emerge and grow quickly tend to be more tolerant to seedling diseases. By the two- to four-leaf stage, the below ground parts of the plant produce a wax called suberin to withstand further infections and are able to regenerate more rootlets than they lose. For quick germination, emergence and vigorous seedling growth, plant high germination certified seed 12 to 25 millimetre (1/2 to one inch) deep into firm, moist, adequately fertilized soil. Waiting until soil temperatures reach 10 degrees Celsius will provide for rapid emergence, but waiting and seeding late may have other risks such as later harvest and reduced yield potential. Learn more about optimizing stands in the plant establishment section.
Disease infection tends to be higher in seedlings stressed due to:
- Deep seeding. This places seed into colder soil, forces it to grow through more soil to emerge, and increases the number of soil pathogens and infested residue particles that come into contact with the seedling.
- Poor seed quality. High germination tests usually correspond with high vigor, which means seed will germinate and grow faster.
- A cold, too wet, too dry, or too crusty seedbed. Together or separately, these factors can slow seed germination and growth, which extends the period where diseases can infect.
- Toxic herbicide residues.
- Flea beetle injury. Damage from flea beetle feeding slows crop development.
- Placement of excessive fertilizer with the seed. Fertilizer placed with the seed may damage plant tissues and delay or reduce germination and emergence, prolonging the period of susceptibility and increasing infection. Avoid inadequate or unbalanced nutrients, which also favor the disease complex fungi.
Rotating canola with non-cruciferous crops can also minimize disease losses. Seedling disease damage may be higher when canola is seeded into canola stubble, and when canola is grown at a high frequency in a long-term rotation.
Genetic resistance
There is no genetic resistance to seedling disease complex available at this time. Genetics could play an indirect role through the effect on early season growth in terms of seed and seedling vigourSeed properties that determine the potential for rapid uniform emergence and development of normal seedlings under a wide range of field conditions. More. However, there is no indication that differences among commercially available cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More are large enough to affect varietyA variety is a variant of a species that evolved in nature without the intervention of humans, e.g. Brassica oleracea variety (in short form, var.) botrytis (cauliflower), var. capitata (cabbage), var. italica (broccoli), etc. More choice based on seedling disease risk.
Footnotes
- Hwang, S.F., Ahmed, H.U., Gossen, B.D., Kutcher, H.R., Brandt, S.A., Strelkov, S.E., Chang, K.F., & Turnbull, G.D. 2009. Effect of crop rotation on soil pathogenA disease-causing organism (such as a fungus or bacteria). More population dynamics and canola seedling establishment. Plant Pathology Journal, 8(3), 106-112. doi: 10.3923/ppj.2009.106.112[↩][↩][↩]
- Yitbarek, S.M., Verma, P.R., Gugel, R.K., & Morrall, R.A.A. 1988. Effect of soil temperature and inoculum density on pre-emergence damping-off of canola caused by Rhizoctonia solani. Can. J. Plant Pathology, 10, 93-98.[↩][↩]
- Hwang, S. F., Ahmed, H. U., Turnbull, G. D., Gossen, B. D., & Strelkov, S. E. 2015. Effect of seeding date and depth, seed size and fungicide treatment on Fusarium and Pythium seedling blight of canola. Can. J. Plant Sci., 95, 293–301. https://doi.org/10.4141/cjps-2014-268[↩][↩]