Weeds, insects and diseases can limit the production potential of canola. To minimize their impact on yields, use an integrated control program or practice pest management that includes a combination of prevention strategies and cultural, physical and chemical controls.
Integrated pest management challenges include diseases (e.g. clubroot, blackleg, sclerotinia, etc.), weeds (identification, timing, volunteers, resistance, rotating herbicide active ingredients, etc.) and insects (identification, unpredictability, accurate determination of thresholds, timing, impact on beneficials, etc.). Economic thresholds exist for each of these pests (weeds, diseases and insects), and their implementation forms the backbone of an integrated pest management plan.
Challenging, changing crop environments continue to require additional research into existing pests and new potential threats to our cropping systems.
An integrated control program
The goal of using integrated pest management (IPM) is to achieve effective management of pests by using all the tools that are available, in the safest manner possible that enhances the sustainability of the farm. IPM uses all the tools that are available for controlling pests, including the use of chemical, cultural, mechanical and biological tools. It does not mean exclusively relying on one technique to manage a pest which in the end can exhaust the technique and render it no long successful in pest management.
IPM is not a new concept. Most canola growers are using several IPM tools. IPM is much more than just scouting a field to assess the pest populations and make a decision of whether to spray or not, and it is rarely useful when practiced in isolation or in the short term. Growers will be more successful with IPM if steps are taken each year to ensure good crop health and to prepare individual IPM practices in each field. There are four steps involved in implementing an IPM program:
- Prevention: Practices that reduce the severity of pest infestation or prevent pest build-up.
- Monitoring and forecasting: Determining what pests are present and what action is required to manage pests.
- Intervention: Actions to reduce the economic crop damage from pests.
- Record keeping: Maintaining a field record system for effective planning.
Underpinning all four of these steps is the grower’s ability to accurately identify pests. By knowing the pest and its lifecycle, growers can plan control programs to exploit weaknesses in pest lifecycles and use the most effective management strategies.
In canola, IPM includes the use of clean healthy vigorous seed, crop rotations, pest monitoring and resistant cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More. It also includes using crop protection chemicals judiciously. IPM aims to minimize the impact of pest on the crop, while maximizing the return from using different pest management tools, in a sustainable manner that takes care of the farm environment.
The biodiversity that comes with crop rotation can be a valuable part of integrated management of insects, weeds and plant diseases. Biodiversity of habitat across the farm can increase populations of bees and beneficials, which can increase yields and provide a natural check on insect populations. Biodiversity through crop rotations can reduce disease severity and expand management options for weeds.
In addition to crop rotations, there are several effective pest management tools farmers can use to improve profitability and achieve their sustainability goals.
Scout and identify before spraying. Spraying has a cost and farmers want to make sure the pest they’re spraying is actually causing enough crop damage to justify that cost. This ‘economic threshold’ is a key part of integrated pest management, but it requires close scouting and counting. Return on investment for scouting and counting comes when planned sprays don’t have to be applied (lower cost) or when an unexpected threat needs immediate action (protected yield). Learn how to put together a robust scouting kit below.

Follow insect thresholds. Established science-based thresholds for many canola insect pests help farmers make better economic and environmental decisions, decreasing the use of prophylactic insecticide applications. The Canola Council of Canada’s Canola Insect Scouting Guide provides a quick reference for insect thresholds. Threshold tables for lygus indicate that if canola is 12 dollars per bushel and spray costs eight dollars per acre, the threshold at the early pod stage is five lygus adults or late instarA developmental stage within one life stage (ex. of an insect) More nymphs per 10 sweeps. Recent work found that in moist and high-yield conditions, it could take many more – up to 50 lygus per 10 sweeps at the early pod stage – to cause an economic two bushel per acre reduction in yield 1.
Know your beneficials. Canola fields are habitat for an incredible diversity of beneficial insects (or ‘field heroes’) such as bees, butterflies, spiders, wasps and beetles. In many cases, these diverse insect groups prey on – or parasitize – insect pests, providing a level of control often exceeding that of chemical means 2,3. Even if farmers don’t identify the beneficial species in their fields, waiting to spray until pest numbers reach thresholds can go a long way to limiting pesticide use and protecting these valuable allies. See examples of beneficial insects in the images below. Find out more about beneficial insects at fieldheroes.ca.

Reduce weed competition early. Early weed control, either through a pre-seed burnoffRefers to a pre-seed glyphosate (herbicide) application that stunts the growth of the perenial weeds in a field (since glyphosate only affects top growth and isn't taken up by the roots in the spring) in order to give the crop (to be seeding shortly after) a better chance at outcompeting the weeds for sunlight, water and nutrients. More or in-crop applications around the two-leaf stage of the crop, is a good way to get more out of the herbicide investment. Farmers can also use narrower rows and higher plant stands to increase crop competition, tank mixCombining two or more crop protection products (that are compatible) in one application. More herbicides to hit weeds with more than one active ingredient, and collect chaff (then feed or burn it) to reduce the weed seedbank.
Manage diseases with genetic resistance and crop rotation. Growers can use pre-harvest scouting to identify diseases present and then use this information to choose seed with resistance to those diseases. Refined testing will also identify the specific blackleg races present in a field, which can be used to make better blackleg-resistance selections. Crop rotation is another valuable integrated approach to disease management, keeping levels low and genetic resistance effective for more years to come.
For information on managing fungicide resistance in canola and other crops, see CropLife Canada’s Manage Resistance Now.
Keep equipment clean. Moving soil means moving clubroot, verticillium, aphanomyces (pea and lentil disease), phytophthera (soybean disease), weed seeds and nematodes. The clean equipment message can also apply to sprayer plumbing. Herbicide residue in sprayers can have a devastating effect on canola, reducing yield and striking a blow to economic and environmental sustainability.
Maintain habitats. Natural habitat has a benefit to farm profitability, including increased crop production efficiency and yields. Setting aside non-profitable areas (where yields don’t justify inputs) will also improve biodiversity, carbon sequestration and land efficiency. A Western Canadian canola study concluded that yield and profit could be maximized when 30 per cent of land within 750m of field edges was uncultivated 4. One of the Canola Council of Canada’s sustainability goals is to increase the percentage of farmland that is seeing an increase in its natural habitat.

Natural spaces and wetlands in close proximity to cropland provide home for beneficial insects and natural enemies, which help manage insect pests and weed seeds. This can reduce input costs, prevent applications of unneeded pest control products, and provide filter strips to prevent runoff of nutrients and chemicals.
The scouting toolkit for pest management
Professional entomologists, plant pathologists, agronomists and growers use many of these items in their scouting toolkits.
- Mobile device: Smart phones and mobility-enabled tablets could be the most valuable scouting tools. They allow us to phone or text from the field for immediate back up or second opinions, including through Twitter and other social media platforms. Apps can help with record keeping and pest identification. GPS functions can pinpoint exact scouting locations. Their cameras are particularly valuable for photographing pests that cannot be identified without further support or reference material or for any other field observations. Take a lot of pictures, from various angles, ensuring the pest in question is in focus. Carry plain white paper to hold behind the pest. This makes it easier get the camera focused on the pest instead of the crop or landscape in the background. Macro (close up) lenses for smart phones help take focused close ups for easier identification. For identification queries, collect these four basic pieces of information for each photo: What crop is the insect in (and where on the plant was it found), location of the field (legal location or GPS if possible), name of the person who collected the sample or took the photo, and the date.
- Seed depth tool: Growers can use the tool to make sure every run of the drill places canola at the recommended half inch to one inch depth. Click here for tips on where and how to check seed depth.
- Hand trowel: Hand trowels are handy for wireworm and cutworm scouting, and trowels with depth markings on them can also be used to determine the depth from which seedlings are emerging while evaluating health of hypocotyls and roots. When scouting for underground cutworms, look above-ground for bare patches, holes or notches in foliage, and clipped plants. Start digging where damaged or missing plants are found. In moist soils, cutworms will stay close to the surface. In dry soils, they may go down eight to 10 centimetres (up to four inches). They may also go deeper in the afternoon heat, so this may not be a great time to scout. To scout, dig up soil from a one square foot area to a depth of 10 centimetres and put it into a basin. Loosen the soil and shake it up to activate cutworms. Repeat a few times throughout the field. A sieve can help separate insects from dry soil. Find a sieve or strainer with holes big enough for your soil type.
- Standard sweep net: Lygus bug and cabbage seedpod weevil economic thresholds are based on sweep net counts, and proper counts depend on using the standard sweep net and technique. The standard size has an 89 centimetre (35 inch) long handle and 38 centimetre (15 inch) diameter net. Lygus can show up in all areas of the Prairies, but even if this area doesn’t typically have lygus or cabbage seedpod weevil, a sweep net can be useful to identify the presence of beneficial insects, diamondback moth larvae and other insect pests. Alberta’s government site has info on where to buy them.
- Large baggies: especially the breathable ones are a handy companion for the sweep net. Flying insects can pop out of the net quickly, making it harder to do an accurate count. Carefully dump all contents into the bag and then count insects through the perforated bag.
Note: Sweep net sampling is notoriously variable. Making a spray decision based on one set of 10 sweeps means a high chance of making the wrong decision. Extension entomologists recommend a minimum of five sampling sites, in a ‘W’ or ‘X’ pattern throughout the field, with 10 sweeps at each site. With lygus in particular, time of day, temperature and wind speed can make a difference in counts. It may be best to scout two days in a row, unless average numbers are well above threshold.
- Magnifying glass/hand lens: A 10X magnifying glass can be used to identify different insect species based on specific markings, to identify very small insects, such as thrips, to spot pycnidiaA type of fruiting body (produced by a pathogen, such as the blackleg-causing Leptosphaeria maculans pathogen) that appears as pepper-like spots (which are spore-bearing structures) within lesions. More in blackleg lesions, and to look at the growing points of frosted canola to see if they’re regrowing.

- Three sided or two sided “square”: Scouting squares are easy to make. Some insect thresholds are based on counts per square foot or square metre, so use a two or three sided “square” to slip more easily into a heavy canopy and help more accurately estimate the area of the counts. A home-made two-sided square, with a grid on each length showing one foot, half metre and full metre lengths will serve multiple purposes. With open sides, the square slips into the canopy more easily than a full square. For bertha armyworm, for example, lay down the square with the half metre length on each side. Remove leaf litter to count berthas on the ground and give canola plants a shake to remove berthas from the plants. Count the berthas that fall into that half metre by half metre square. Multiply by four to get the count per square metre. This Canola Watch quiz has more scouting tips.

- Hoop or metre stick: The standard 56 centimetre-diameter hoop is equal to 1/4 of a square metre.
Plant counts can help assess whether the stand has reached the optimum density of 50-80 plants per square metre (five to eight per square foot), and whether these stands are uniform throughout the field. This can help with reseeding decisions and seeding rate assessment, and in future decisions about pest threats and whether thresholds should be lowered if plant stands are at critical low levels. This Canola Watch articles shows how to do counts with a metre stick or hoop.

- Clippers: Clippers for cutting stems to check for blackleg. To check fields for incidence and severity of blackleg, clip stems just before swathing and look at their cross sections. Clippers provide a nice clean cross section, better than a jack-knife. Splitting stems by hand is too difficult and destructive to provide a good cross section. Spend the money on a good set that can cut through a large canola stem cleanly. Buy stainless steel (avoid carbon steel) so they can be disinfected and will not rust.
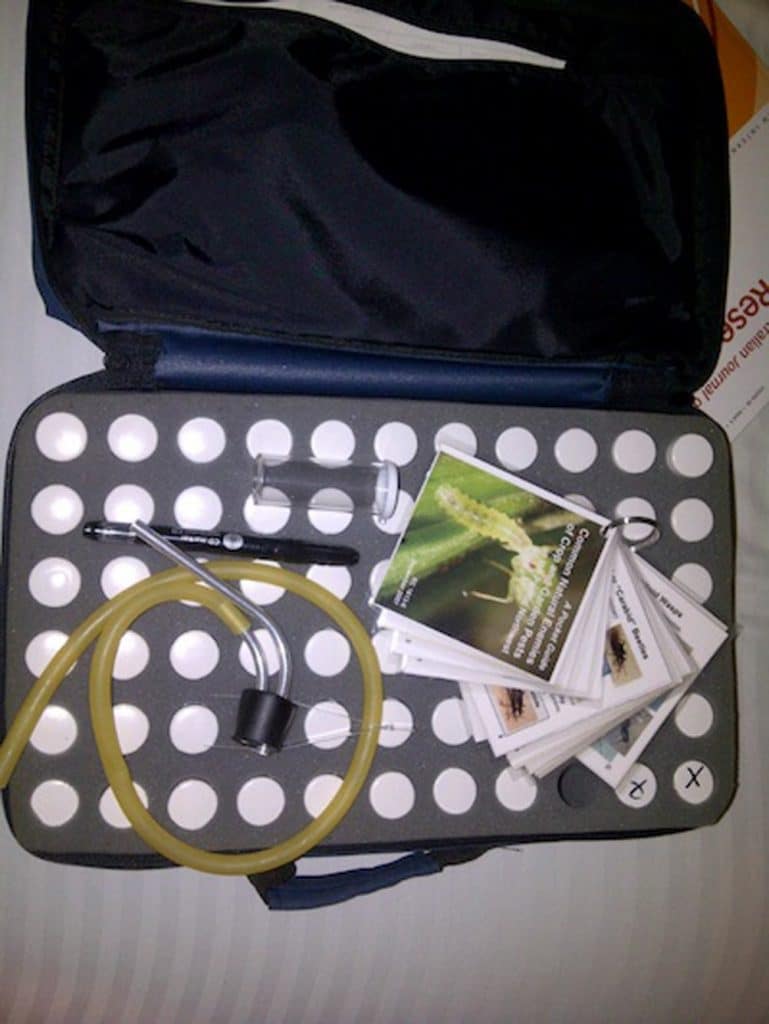
- Containers for samples: This is an elaborate insect collector’s kit with numerous small vials and an aspirator. Growers and agronomists scouting fields are often the first line of defense when it comes to identifying first arrivals each year and identifying new insect pests. But this defense is useless if the insect can’t be identified. Vials or sturdy plastic containers will keep insects in good shape while in transport to the lab.
- Bags: Plastic baggies with perforations work really well for grasshoppers. Paper bags work for keeping samples of stems covered with aphids. Paper bags are better for plant samples for disease identification because paper avoids mold and decomposition that can occur in plastic.
- Aspirators: can help suck insect specimens off a plant and put them into the vials. An aspirator plugs into the top of the vial, and has hoses going in and out. Sucking on one end and holding the other end up to insect, vacuums them up the hose and into the vial. This is faster than tweezers and does less damage to the insect making identification easier.
- Booties: Booties to prevent spread of soil on boots. Biosecurity is important for anyone moving from field to field and farm to farm. Mud on the bottom of boots can spread noxious weed seeds and clubroot spores. The amount of soil required to initiate clubroot infection in a new field is unknown. Therefore, any soil transfer from an infested field should be viewed as a risk. Growers and agronomists and anyone else who walks fields is encouraged to use disposable booties, regardless if there is confirmed clubroot in their region (often clubroot is only found after there is a fairly established infestation). Veterinary offices often have booties for sale. The blue booties used in the photo above are from Vetsource. Use one set of booties per field, and dispose of used ones properly. If rubber boots are worn without booties, they will need to be scraped clean, washed (washing can be done in a 20L pail or a Rubbermaid type container with lid) and disinfected before the next visit. Include a spray bottle of two per cent bleach solution for cleaning boots and equipment between fields and garbage bags for disposal of booties or to wrap contaminated equipment.
- Bleach: Use only sodium hypochlorite. Target a two per cent concentration of bleach to water (about 50/50 blend of regular four per cent household bleach and water). Do not buy bleach unless the jug specifies sodium hypochlorite. Bleach degrades fairly quickly, so purchase quantities that can be used quickly. Discard bleach after the bottle has been open for 3 months. Check best before dates for unopened bottles. Use two per cent bleach solution in a boot bath and for misting bottles to sanitize tools. Other scouting kit tools to clean gear between fields can include wire brushes and scrapers, disinfectant wet wipes and boot baths. Why use bleach and not another disinfectant?
- Flags: If an area with suspicious weeds, disease lesions, or building insect populations is found, it can be helpful to mark them so that same spot can be returned to later to monitor the potential problem. Plastic stemmed flags are preferred in case they are forgotten in the field and accidentally cut them with the swather.
- Gloves: Nitrile or latex gloves should be part of your biosecurity kit, and can keep your hands clean when handling insects, plant roots, etc.
- Beating sheet: This is an expandable white sheet made from plasticized material. It has cross pieces underneath to keep corners square. Entomologists often have these on hand for field days, to bang out aphids, caterpillars or other insects to do accurate counts. For growers, a piece of cardboard or truck hood or tail gate are often handier and can be just as effective.
- Cooler or insulated bag: Keeping insect or plant samples in good condition is much easier in cool conditions. Even without an icepack, the closest gas station likely has ice or something frozen that can be used for an icepack.
- Random essentials: A notebook and pen, either the real thing or an electronic version, is handy to record details of areas to return to for monitoring, noting distribution of disease, sampling, etc. Also have a grease pencil or Sharpie on hand to mark containers. Tape, scissors and zip ties are also handy.
- Resource material –
- Keep Canola Council of Canada pest management publications and a link to the Canola Encyclopedia on hand for identification of disease, pests and deficiencies.
- AAFC’s Guide to pest wireworms in Canadian Prairie field crop production
- AAFC’s Cutworm Pests of Crops on the Canadian Prairies
- AAFC’s Field Crop and Forage Pests and their Natural Enemies in Western Canada guide
- Field Heroes scouting guides
Table 1. Careful use of crop protection chemicals
Table 2. Scouting, economic thresholds and record keeping
For details on the application of integrated pest management strategies to specific insects, canola diseases and weeds, see the Canola Encyclopedia sections on insects, diseases and weeds.
Footnotes
- Cárcamo, H. (2016). Updating economic thresholds for lygus bugs in canola in Alberta, Canada. 10.1603/ICE.2016.115245[↩]
- Giberson, D., & Cárcamo, H. 2014. Arthropods of Canadian Grasslands. Vol 3: Biodiversity and Systematics Part 1[↩]
- Giberson, D., & Cárcamo, H. 2014. Arthropods of Canadian Grasslands. Vol 4: Biodiversity and Systematics part 2 DOI: 10.3752/9780968932179[↩]
- Morandin, L. 2013. Landscape patterns environmental quality analysis- Landscape patterns project. Government of Alberta.[↩]







