The root rot complex is mainly caused by three types of soil-borne pathogens which affect the roots of adult plants. The disease symptoms are potentially serious, and the incidence, as well as intensity, is increasing throughout the Prairies. An understanding of the disease cycles, prevention strategies, ability to identify symptoms and manage them will improve the potential for successful canola crop.
Important tips for best management of root rots
- Use B. napusAlso referred to as Argentine canola, it is the species of canola currently commonly grown in Canada. More cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More where possible, as they are relatively less susceptible than B. rapaAlso referred to as Polish canola, it is the less commonly grown species of canola currently grown in Canada. More and B. junceaAlso referred to as brown mustard, it is a minor crop (from the Cruciferae or Brassicaceae plant family, commonly known as the mustard family) grown in Canada. More cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More. Plant breeders are making good progress on the development of B. rapaAlso referred to as Polish canola, it is the less commonly grown species of canola currently grown in Canada. More cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More with resistance to brown girdling root rot.
- Use recommended seed treatments for control of the root rot complex, however, these fungicides do not provide season-long control.
- Use balanced fertility and proper seeding depth to help guard against root rots; weaker plants are more susceptible than healthy robust plants.
Overview
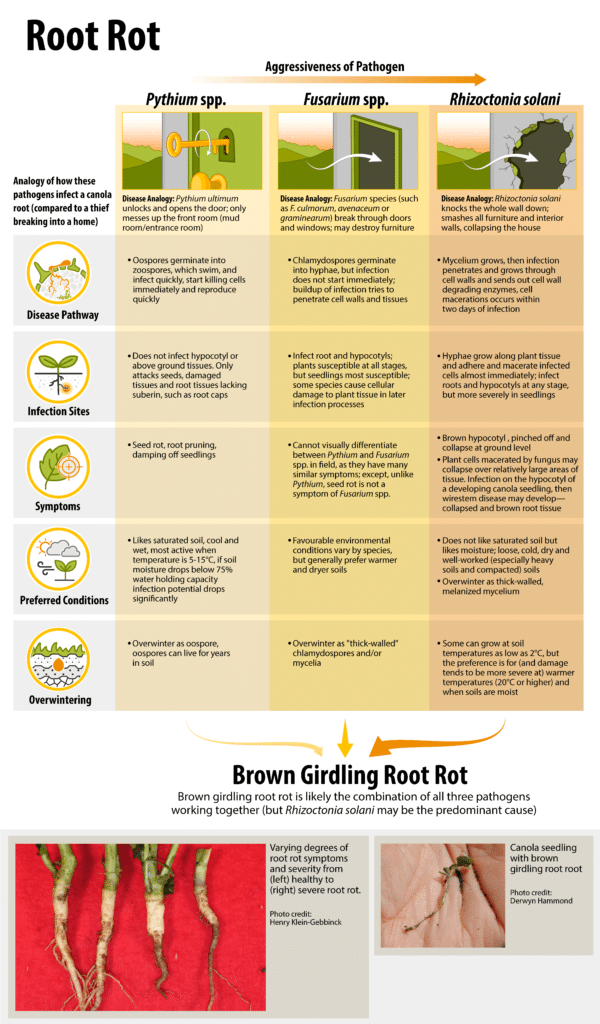
Rhizoctonia solani, Fusarium species and Pythium species are the primary pathogens in the root rot complex, which includes foot rot, root rot and brown girdling root rot (BGRR). (See Seedling disease complex).
The pathogens causing these symptoms are potentially serious, and the incidence, as well as intensity, is increasing throughout the prairies. Brown girdling root rot is the most serious root rot disease and is believed to be caused primarily by the fungus Rhizoctonia solani, with secondary infections by Fusarium spp. In most of Western Canada, these diseases manifests primarily at the seedling stage, while in the Peace River region both seedling loss and the much more serious BGRR occur 1.
Brown girdling root rot affects all cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More, although B. napusAlso referred to as Argentine canola, it is the species of canola currently commonly grown in Canada. More (Argentine) cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More are more resistant. Losses are highest when wet soil conditions occur at early flowering, followed by dry weather later in the season. Infection levels may reach 80 to 100 per cent in some fields, with losses approaching 50 per cent. In the 2010 disease survey BGRR was found in 20 per cent of canola crops in Alberta with a mean incidence of three per cent ((The Canadian Phytopathological Society. 2011. Canadian Plant Disease Survey: Disease Highlights. Retrieved from http://phytopath.ca/download/cpds-archive/vol91/cpds_vol_91_2011.pdf)). The regions with the highest prevalence (percentage of fields affected) of BGRR were in the Peace River region, with some fields having incidences (percentage of plants infected) as high as 60 per cent.
The Agriculture and Agri-Food CanadaAgriculture and Agri-Food Canada is a department of the Government of Canada. More (AAFCAgriculture and Agri-Food Canada is a department of the Government of Canada. More) Beaverlodge, AB Research Centre found that nearly half of the microorganisms isolated from BGRR-infected root tissue were identified as Rhizoctonia solani 2. Rhizoctonia solani exists as several strains with anastomosis group AG2-1 commonly identified in the Peace River region and the less virulentA form which causes infection (and can result in a disease) More AG4 in other regions. The other diseases in this complex, foot rot and root rot, can be caused by any of these pathogenA disease-causing organism (such as a fungus or bacteria). More and often are less common and less severe as BGRR.
Disease descriptions
Brown girdling root rot
The brown girdling root rot (BGRR) pathogens are soil-borne. Light brown lesions with irregular margins first appear at or after the onset of flowering, usually 7.5 centimetres (three inches) or more below the soil surface on the taproot or main lateral roots. Later lesions may appear anywhere on the taproot. As the lesions develop they expand, grow together, become sunken and eventually girdle the taproot, causing poor pod development, shriveled seeds and uneven maturity. The sunken lesions become dark brown. Roots below the girdling location rot off. The lesion continues to develop upwards, sometimes to the soil surface but never moves into the stem. Root tissues above the infected part become swollen.
In moist conditions, the whole taproot may be destroyed up to the soil surface. Girdled plants may survive and even set some seed if they are not uprooted or blown over by wind.
In dry soil, a sound taproot stub remains. The above ground parts of the plant remain turgid as long as there is any root connection with moist soil. Taproot stubs that retain a few roots are sometimes capable of regenerating main laterals as well as fibrous roots. Plants with girdled taproots wilt, dry up and shrivel, even though soil moisture may be adequate for plants with normal root systems.
Yield losses will depend on the amount of root system lost by girdling. If only root stubs are present or brown lesions girdle the taproots, the disease will result in considerable yield loss. If brown spots are present, but do not girdle the root, actual disease losses may be minimal. Losses are a result of increased pod sterility, loss of seed weight, and seed shriveling and plant death from desiccation or wind toppling. Girdled plants that survive ripen prematurely and tend to be pulled out of the ground rather than cut during swathing, increasing shatter losses.
Disease development appears to be favoured by fine-textured heavy clay soils with high levels of copper (four to 20 parts per million). The Beaverlodge, Alberta AAFCAgriculture and Agri-Food Canada is a department of the Government of Canada. More Research Centre found that disease severity was similar for all tillage systems 2. Brown girdling root rot can be more severe in canola crops that are grown after canola (in a rotation), clover or fescue than in canola crops that follow summerfallow. For reasons not clear, decomposing fescue sod is conducive to the development of this disease. It is unpredictable because it can also be severe where canola has never been grown previously, and even on freshly broken land that has never been cropped. Several cruciferousPlants belonging to the family Brassicaceae or (previously referred to as) Cruciferae. More weeds, including stinkweed, shepherd’s purse and ball mustard, also suffer from root rot.
A 2000 study conducted at the AAFCAgriculture and Agri-Food Canada is a department of the Government of Canada. More Research Centre in Beaverlodge, Alberta showed that average seed yield was not reduced on plant roots with non-girdling lesions and superficial non-sinking girdling lesions, but was reduced by 17 per cent on plant roots with girdling and sinking lesions, and by 65 per cent on plants with decayed taproots. Over two years, yield losses in growers’ fields ranged from one to five per cent 3.
Foot rot
In the growing season, brown, hard, clearly defined lesions occur near the stem bases of canola plants. The lesions may be black-bordered, and during periods of humid weather, pink spore masses may develop on diseased root tissues. Discolouration of the upper part of the taproot above the lateral roots may occur. In severe cases, the stem is girdled, killing the plant. Yield loss may occur when the stem is one-half girdled. Yield losses are light when lesions occur late in the season. Affected plants may ripen prematurely as scattered plants or in patches in the field. Where foot rot develops late in the season, the earlier maturing B. rapaAlso referred to as Polish canola, it is the less commonly grown species of canola currently grown in Canada. More cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More may escape infection or lesions may not have time to develop to the point that yield is seriously reduced.
Root rot
The root rot pathogens are soil-borne. Losses are highest when wet soil conditions occur at early flowering, followed by dry weather later in the season. The root rot complex disease causes brown lesions on the main taproot near the time of flowering.
Disease cycles
Pythium species
The Pythium genus which causes root rots in canola is not a true fungus, but rather an oomyceteA type of microbe that is somewhat similar to fungi, but in its own kingdom. Species of the Pythium genus (which causes root rots in canola) is an example of an oomycete. More. Pythium favours saturated soil and is most active at soil temperatures of five degrees Celsius to 15 degrees Celsius. Once soil moisture drops below 75 per cent water holding capacity, infection potential really drops. Pythium tends to be the first to infect, getting at the seed within three to five days of germination.
There are several species of Pythium that infect canola roots, but the most prevalent appears to be P. ultimum. Pythium species are generalist pathogens infecting many species of plants (therefore while a diverse rotation can be beneficial, it won’t eliminate this soil-borne disease). They are also saprophytes, or decomposing organisms, although they are not as aggressive as other saprophytic microbes.
Pythium’s life cycle begins each year overwintering as an oospore, a hardy survival structure. These oosporesA hardy survival structure of an oomycete. Species of the Pythium genus (which causes root rots in canola) overwintering as an oospore. More germinate in cool wet soils into flagellated zoospores which swim in soil water towards canola seeds and roots attracted by root exudates. These zoospores take advantage of damaged plant tissue to cause infection, which is commonly found in newly developing seedlings and lateral roots. Zoospores cluster around roots, producing germ tubes and appressoria, which degrade plant cell walls to infect the epidermisThin outer layer of a tissue (such as the thin outer later of plant tissue which peels back from canola stems which are infected with verticillium stripe). More of canola roots. Once an infection is initiated, this pathogenA disease-causing organism (such as a fungus or bacteria). More then quickly produces myceliumMicroscopic fungal filaments More which grows intra- and intercellularly through the surrounding root tissue. Within four days after the infection is initiated, it will produce reproductive structures called sporangia, which in turn produce new zoospores that then infect new root tissue. Within a few weeks this pathogenA disease-causing organism (such as a fungus or bacteria). More will produce new oosporesA hardy survival structure of an oomycete. Species of the Pythium genus (which causes root rots in canola) overwintering as an oospore. More, completing the cycle.
Fusarium species
The Fusarium genus has many species which are fungal plant pathogens and there is a range of variability between species and within a species. F. avenaceum, F. oxysporum, and F. culmorum have been found to cause root rots in canola 4, although other species may also be involved. While many species favour warmer temperatures and moist soils, there is variability between the environmental factors that favour one species over another in infecting plants. Therefore under almost any type of environmental condition some Fusarium species will be able to cause infection. The severity of the disease may be greater in wet or dry years when crops are already vulnerable.
Fusarium species overwinter as thick-walled chlamydospores. These spores germinate in the spring into myceliumMicroscopic fungal filaments More, which seek out developing canola roots to infect. When Fusarium myceliumMicroscopic fungal filaments More come into contact with root tissue, the fungus produces an infection cushion that allows the pathogenA disease-causing organism (such as a fungus or bacteria). More to invade the root tissues. Depending on the species involved, this fungus then grows intracellularly, infecting a range of tissues (and producing symptoms), with some invading vascular tissue growing up into the base of the stem producing foot rot symptoms. Some Fusarium species are more aggressive than others. Although many Fusarium species produce airborne spores (conidiaSingle-cell spores or fungal propagules (such as those Verticillium species form). More and ascosporesMicroscopic asexual spores that typcially become airborne. More) these do not play a role in canola diseases since stem tissue is not susceptible to Fusarium infection. As plants mature, myceliumMicroscopic fungal filaments More differentiates asexually into chlamydospores to survive the winter months.
Rhizoctonia solani

Rhizoctonia solani is a fungal pathogenA disease-causing organism (such as a fungus or bacteria). More that infects many species of plants and lives as a successful saprophyte (decomposing organism). This pathogenA disease-causing organism (such as a fungus or bacteria). More causes one of the more familiar of the canola seedling disease symptoms, the collapsed and brown root tissue called wirestem. The Canadian Prairies has two common groups of this species. One group, which pathologists call AG2-1, favours cold to cool conditions, while the other group, AG4, favours cool to warm conditions. AG2-1 appears to be more successful at infecting over a wider range of temperatures than AG4) 5">https://doi.org/10.1016/0261-2194(92)90072-D)). AG2-1 is more of a crucifer specialist and is the more common group in the Peace region. It can be active at soil temperatures as low as two degrees Celsius, and it tends to be more severe when soils are moist and warmer than two degrees Celsius. Like root diseases in general, damage tends to be worse in heavy soils and compacted soils.
Instead of hardy spores, R. solani overwinters as melanised myceliumMicroscopic fungal filaments More, often in association with decaying organic matter in the soil. When soil warms, this myceliumMicroscopic fungal filaments More grows through the soil and comes into contact with canola roots and hypocotyls. The hyphae of this myceliumMicroscopic fungal filaments More grow along the canola tissue surfaces producing multiple infection cushions along its length, allowing this pathogenA disease-causing organism (such as a fungus or bacteria). More to infect in multiple locations at once. Plant cells are macerated by the fungus may collapse over relatively large areas of tissue. If this infection is on the hypocotyl of a developing canola seedling, then wirestem disease may develop. Root infections can lead to root rots and BGRR. Decomposing canola tissue acts as a source for R. solani myceliumMicroscopic fungal filaments More to melanise to survive the next winter.
See this Canola Watch article for a good analogy of the three different families of seedling disease complex.
Influence of environment
The root rot pathogens are soil-borne. Losses are highest when wet soil conditions occur at early flowering encouraging extensive root rot development, followed by high temperatures and dry windy conditions. Root rot complex diseases are destructive, but sporadic, and sometimes linked to root maggot infestations or high rainfall and waterlogged soil, particularly during flowering.
Identification
When scouting for the cause of poor emergence, check for the following symptoms of seed decay, stem rots and root rots that are all part of the seedling disease complex.
- Seeds fail to germinate and become soft and pulpy.
- Seeds germinate but the developing seedlings decay and fail to emerge.
- Seedlings emerge and appear normal above ground, but either the roots decay with rot moving rapidly up into the hypocotyl, or the young stem (hypocotyl) may be partially or completely girdled with decay and shrivel at any point. In this case, the young above-ground parts of the canola seedling may also wilt and exhibit a purplish or chlorotic discolouration. When the decay reaches the soil surface, the emerged part of the seedling topples over, wilts and dies.
- The hypocotyl appears constricted or shriveled and may be discoloured reddish brown. In moist topsoil, the shriveled stem may persist for a while, but in dry windy conditions, the whole seedling disappears in a few days. Seedlings may also emerge and then stagnate in the two- to four-leaf growth stage even when growing conditions appear favourable. Root hairs appear to be missing.
Symptoms specific to each seedling root rot should also be considered.
Brown girdling root rot
Early symptoms consist of light-brown lesions on the taproot or main lateral roots well below the soil line. These enlarge and coalesce, become sunken and girdle the taproot. Only a short taproot stub may be left. Plants ripen prematurely in the field, often before any seed has been set. Girdled plants are subject to death from desiccation or uprooting by wind. Other symptoms include:
- Light brown lesions on taproot and at bases of larger roots
- Taproot finally girdled, leaving a stump
Foot rot
To correctly identify this disease, look for hard brown lesions at the stem base and salmon-coloured spore masses may be present in lesion. Losses tend to be minor as lesions develop late in season. Early lesions can cause premature ripening and reduced yields
Root rot
The symptoms are variable in colour and shape but can be grouped into four general types:
- a light grey oval lesion of the upper taproot
- a dark grey discolouration of the lower taproot and internal tissue, later becoming black
- a light brown, soft, diffuse taproot lesion
- a dark brown, sunken, sharply defined taproot lesion
Affected regions
Root rot symptoms occur sporadically throughout canola growing areas. Brown girdling root rot is an extremely widespread in the Peace River region of Alberta and British Columbia, but occurs only occasionally in the northern parkland zone. It tends to be a more serious problem in B. rapaAlso referred to as Polish canola, it is the less commonly grown species of canola currently grown in Canada. More cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More as they have greater susceptibility. In the Peace River region, BGRR causes greater yield losses than all other diseases.
Testing to confirm
Brown girdling root rot is rarely seen outside of the Peace region, so tests will need to be done to confirm.
Prevention
Crop rotation
Risk of root diseases can increase with increasing canola cropping intensity. Consider including field peas in the rotation (to improve the nitrogen level) along with other pulse or cereal crops to reduce disease severity.
Field management
Consider these cultural control measures to prevent root rot:
- Control volunteer canola and cruciferousPlants belonging to the family Brassicaceae or (previously referred to as) Cruciferae. More weeds in rotation to help prevent a build-up of root pathogens in the soil.
- Use clean seed.
- Maintain recommended nitrogen, phosphorus, potassium and sulphur fertility levels in the soil. Nitrogen decreases disease severity. Liming the soil may also be beneficial in reducing disease severity.
- Use B. napusAlso referred to as Argentine canola, it is the species of canola currently commonly grown in Canada. More cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More since they are only moderately susceptible to root rot, compared to the more susceptible B. rapaAlso referred to as Polish canola, it is the less commonly grown species of canola currently grown in Canada. More cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More.
- Use the management practices outlined for the seedling disease complex to establish a vigorous, uniform growing crop.
Management
Chemical control
- Use a recommended seed fungicide treatment to control the seedling disease complex stage of these diseases.
- No economical chemical controls are available for BGRR.
Genetic resistance
B. rapaAlso referred to as Polish canola, it is the less commonly grown species of canola currently grown in Canada. More (Polish) cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More are generally more susceptible to BGRR. Use B. napusAlso referred to as Argentine canola, it is the species of canola currently commonly grown in Canada. More (Argentine) cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More in suitable climatic regions, as they are only moderately susceptible, but under the right conditions BGRR can still be a problem.
Plant breeders are making progress on the development of B. rapaAlso referred to as Polish canola, it is the less commonly grown species of canola currently grown in Canada. More cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More with resistance to BGRR. Researchers at AAFCAgriculture and Agri-Food Canada is a department of the Government of Canada. More Research Centre in Beaverlodge, Alberta continue screening B. rapaAlso referred to as Polish canola, it is the less commonly grown species of canola currently grown in Canada. More lines for resistance in the field.
Footnotes
- Woods, D. L., Turkington, T. K., McLaren, D., & Davidson J. G. N. 2000. Breeding summer turnip rape for resistance to brown girdling root rot. Can. J. Plant Sci., 80, 199-202.
- Soon, Y. K., Klein-Gebbinck, H. W., & Arshad, M. A. 2005. Residue management and crop sequence effects on the yield and brown girdling root rot of canola. Can. J. Plant Sci., 85, 67-72.
- Klein-Gebbinck, H. W., & Woods, D. L. 2002. Yield loss assessment in canola: Effects of brown girdling root rot and maggot damage on single plant yield. Plant Dis., 86, 1005-1010.
- Fernandez, M. R. 2007. Fusarium populations in roots of oilseed and pulse crops in eastern Saskatchewan. Can. J. Plant Sci., 87(4), 945–952. https://doi.org/10.4141/P06-145
- Kataria, H.R., & Verma, P.R. 1992. Rhizoctonia solani damping-off and root rot in oilseed rape and canola (Review). Crop Protection, 11(1): 8-13.